
Aims for the SHIELD Doctoral Network
The SHIELD doctoral network (DN) aims to substantially advance our understanding of IAIs beyond the current state-of-the-art, from their multifactorial pathogenesis to new biomaterials with antibacterial properties, bridging the gap between basic research and practical applications through translational research. This DN is structured around three scientific pillars (SPs, areas of scientific focus) that drive research across three clinical areas, orthopaedics, otology and odontology, where infection control strategies are needed.
The SHIELD doctoral network (DN) aims to substantially advance our understanding of IAIs beyond the current state-of-the-art, from their multifactorial pathogenesis to new biomaterials with antibacterial properties, bridging the gap between basic research and practical applications through translational research. This DN is structured around three scientific pillars (SPs, areas of scientific focus) that drive research across three clinical areas, orthopaedics, otology and odontology, where infection control strategies are needed.
These pillars are:
SP 1) Regenerative medicine: developing therapeutic strategies that support healing and tissue regeneration in the context of IAIs.
SP 2) Biomaterial science: design and development of innovative biomaterials that minimise bacterial adhesion and biofilm formation on medical devices.
SP 3) Translational research models: bridging basic research with experimental proof-of-concept by utilising in vitro models to study bacterial behaviour and cellular host responses, advancing to in vivo models that mimic clinical scenarios of IAI.
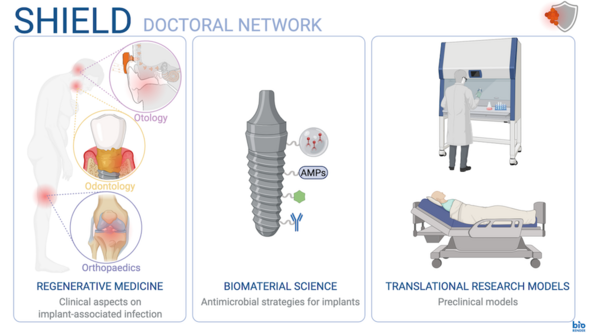
Each clinical area within SHIELD focuses on three core methodological approaches (MAs):
MA 1) Investigation of clinical aspects of IAI conducting clinical studies within each area to gain scientific knowledge on its pathogenesis and optimise preventive and management practices for infection control.
MA 2) Antimicrobial strategies for implants, including research & development and gateway to industry: a) Development of new coatings and surface modifications of implants that prevent bacterial adhesion and biofilm formation, thereby improving their durability and safety. b) Exploring bacteriophage-based therapies to combat antibiotic-resistant bacterial infections in implants. c) Functionalisation of implants with AMPs to enhance implant integration and reduce infection risk.
MA 3) Development of preclinical models to study IAI mechanisms and provide proof-of-concept of the antimicrobial implants and therapies.